The term water vapour pressure is used to quantitatively describe the water vapour content of the atmosphere at any given moment. Recall from Lesson 3 that temperature is the average kinetic energy of a substance. With this idea in mind click here to view a graph of the change in water vapour pressure as temperature increases. How are temperature and water vapour pressure related?
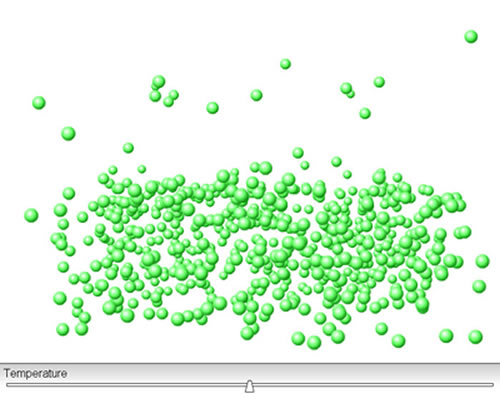
Click on the image above. Move the temperature slider bar to increase the temperature. Watch what happens. How does the dynamic equilibrium between particles in the liquid and gas phases change as temperature increases?
As the temperature increases, larger numbers of particles gain enough kinetic energy to escape into the vapour phase and fewer particles slow down enough to rejoin the liquid phase.
Question: Refer to the graph of water vapour and temperature here. How would you expect an increase in global average temperature from 14-16 degrees Celsius to affect the amount of water vapour in the atmosphere